Understanding the Connection Between Gases and Active Kids
Written on
Chapter 1: The Nature of Gases
Gases consist of molecules that are constantly in motion, colliding with one another. Take air as a prime example; it primarily comprises nitrogen, oxygen, water vapor, argon, and carbon dioxide. At the molecular level, these different types of molecules engage in frequent collisions, much like bumper cars in an arcade game. But what drives this perpetual motion among gas molecules?
Molecular movement is fundamentally influenced by temperature. As temperature rises, molecules gain energy and move more rapidly, colliding with one another more frequently. Picture molecules at a high temperature as energetic toddlers in a small room after indulging in a lot of candy—bursting with energy and darting about erratically.
Conversely, when temperatures drop, molecular activity slows down, resembling toddlers who are worn out after running around for half an hour.
Absolute Zero
You might wonder if molecules come to a complete standstill as temperatures continue to decrease, much like exhausted toddlers taking a nap. This brings us to a specific temperature known as absolute zero, which is the lowest possible temperature at -273°C or -459°F. Absolute zero forms the basis of the Kelvin temperature scale, where 0 K is equivalent to -273°C.
Properties of Gases
What defines a gas? When your air conditioning unit blows air, it occupies a particular space, indicating one of the fundamental properties of gases: volume. Consider filling your car's tires with air; you do so to achieve a specific pressure, another key property. Temperature is the third property, while the number of molecules within the gas is the last. These properties—pressure, volume, temperature, and molecular count—are interrelated, as illustrated by the ideal gas law.
Volume Explained
Volume refers to the amount of space an object occupies, a seemingly straightforward concept. However, students often find themselves confused when distinguishing between volume, area, and length.
To clarify, length is measured in meters (m), area in square meters (m²), and volume in cubic meters (m³). You may also encounter centimeters (cm) or inches (in), which are commonly used in the US; for reference, 2.54 cm equals one inch.
Let's consider a classroom measuring 6 meters in length. Imagine it is suddenly overrun by cow activists, with each cow being about 2 meters long. If cows line up against the back wall, how many can fit? The answer is three cows (6m / 2m = 3). This is an example of a length problem.
Now, suppose even more cows join the protest, and they decide to fill the classroom entirely, assuming they are square cows measuring 2m x 2m. In this case, the total would be 9 cows, calculated as 3 x 3.
Finally, if the cows were to stack on top of each other as much as possible, let's say they are cubic cows of dimension 2m x 2m x 2m. How many can fit now? The answer is 27 cows (3 x 3 x 3). That's a significant increase compared to just 3 cows at the back!
This discussion about cows highlights a common misconception among students regarding unit conversions. While it's understood that 100 cm equals 1 m, the same does not apply to area or volume. For instance, 100 cm² is not equivalent to 1 m², and 100 cm³ is not equal to 1 m³. To clarify, 100 x 100 cm² equals 1 m², and 100 x 100 x 100 cm³ equals 1 m³. Think of it this way: in a length of 1 m, you can fit 100 cm; in an area of 1 m², you can fit 10,000 cm-sized squares; and in a volume of 1 m³, you can fit 1,000,000 cm-sized cubes.
The first video titled "What is a Gas?" explores the fundamental characteristics of gases, including their behavior and properties.
Pressure
A common misunderstanding is equating pressure with force. While related, pressure is defined as force divided by area. For example, consider lying on a bed of nails versus standing on it. The gravitational force remains constant in both scenarios, but the pressure experienced while standing is significantly greater, which can cause skin to break. This difference can be quantified, revealing that pressure from standing can be nearly 50 times greater than when lying down.
To understand pressure mathematically, remember that force (in Newtons, or N) equals mass (in kg) multiplied by gravity (approximately 9.8 m/s² at sea level). Therefore, if force is measured in N and area in m², the resulting pressure is expressed in Pascals (Pa).

Temperature
As previously mentioned, temperature is directly linked to molecular motion, setting the energy levels of these molecules. Thermal energy, which dictates the speed of molecular movement, can be expressed mathematically.
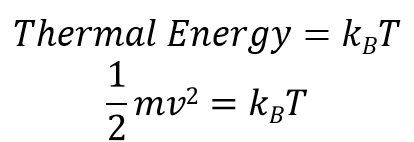
The unit for energy is Joules (J), with T representing temperature in Kelvin and k denoting the Boltzmann constant, an extremely small value of 1.38 x 10⁻²³. While this value may seem negligible at larger scales, it plays a critical role at the microscopic level, leading to observable molecular movements.
Ideal Gas Law
The ideal gas law serves as a vital equation linking pressure, volume, and temperature.
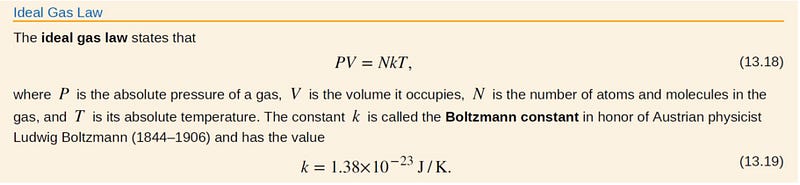
Visually, the relationship between pressure and temperature can be understood as directly proportional; as temperature increases, so does pressure, and vice versa.
The second video titled "Science & Tech | Solid, Liquid, & Gas Experiment" illustrates practical experiments demonstrating the properties of different states of matter.
In conclusion, the ideal gas law encapsulates how pressure and volume are inversely related. As the volume increases, pressure decreases, and vice versa. For instance, within a tire, as molecules speed up due to rising temperature, they collide with the surface more often, raising pressure. If the tire's volume expands, it takes longer for molecules to reach the edges, resulting in lower pressure. Conversely, adding more air increases the number of molecules, elevating pressure to match the gauge reading.
For simulating gases, I utilized LAMMPS, an excellent open-source C++ package for molecular dynamics simulations. The gifs in this post were generated using OVITO. Feel free to reach out if you find this topic intriguing. Follow this publication for deeper insights into material science and the various phenomena shaping our everyday experiences!