generate a comprehensive guide on Karyotyping for Genetic Diagnosis
Written on
Chapter 1: Understanding Karyotyping
Karyotyping is a specialized genetic examination utilized to identify chromosomal irregularities. These abnormalities can manifest as conditions such as trisomy (an extra chromosome, notably seen in Down syndrome), monosomy (a missing chromosome, as in Turner syndrome), or the alteration of chromosomal segments through duplication, deletion, or translocation. This diagnostic method aids in understanding developmental syndromes, evaluating cancer risk, and providing insights for prenatal risk assessments, applicable to individuals of all ages.
This paragraph will result in an indented block of text, typically used for quoting other text.
Section 1.1: Sample Collection for Karyotyping
Samples for karyotyping can be sourced from amniotic fluid or chorionic villus to gather fetal chromosomal data, or from a variety of specimens in children and adults, including tumor biopsies, blood, or bone marrow. The cells are cultured and subsequently halted during mitosis — specifically at metaphase through the use of colchicine. Following this, the nuclear membrane is disrupted to release the DNA, which is then fixed and stained for visualization of chromosome structures.
For a complete overview of the methodology, click here.
Subsection 1.1.1: The Karyotyping Process
The chromosomes are systematically sorted and organized according to a global standard known as the cytogenetic location. This process takes into account the quantity, pairing, shape, and size of the chromosomes in relation to expected norms. The resulting image is referred to as a karyogram. Different staining techniques are employed based on the chromosomal features being examined — for instance, Giemsa staining (G-banding) helps identify larger chromosomal issues. However, smaller microdeletions may not be visible in standard karyotypes.

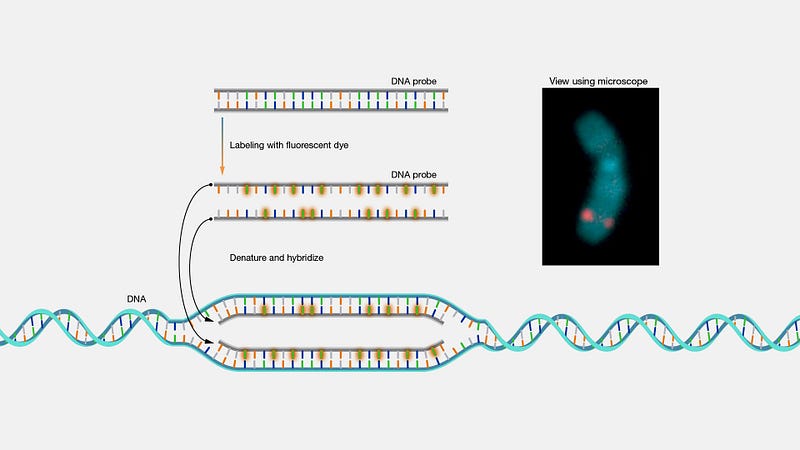
Section 1.2: Limitations of Karyotyping
Karyotyping involves examining a large number of cells to create a karyogram. Despite its utility, one limitation is its inability to detect every chromosomal abnormality. To enhance the analysis, additional modern cytogenetic techniques are often employed. For example, chromosomal microarrays can identify microdeletions that standard karyotyping might miss. Techniques such as Fluorescent In Situ Hybridization (FISH) and Comparative Genomic Hybridization are also useful for examining specific loci or genes on the chromosomes. FISH is frequently utilized for gene mapping.
Chapter 2: Interpreting Karyotypes
A karyotype describes the chromosomal composition of an individual’s cells, denoted as: #, sex + abnormality. For humans, the standard is 46 chromosomes (in 23 pairs). One of these pairs consists of the sex chromosomes, which can be either XX (female) or XY (male). A typical karyotype is represented as 46,XX or 46,XY. For example, a person with monosomy X (missing a second sex chromosome) would be labeled 45,X. In cases of sex chromosome trisomy, such as in Klinefelter syndrome (an additional X chromosome), the karyotype appears as 47,XXY. Similarly, Down syndrome is recognized as a trisomy of chromosome 21, denoted as 47,XX+21 or 47,XY+21. There is a wide array of karyotypes beyond the "normal," each with specific implications based on the chromosomal variations present.
If you require a chromosome analysis or wish to determine your karyotype, consult your physician, who can forward a blood sample to a qualified laboratory.
The first video, "Everything you Need to Know: Chromosome Analysis (Karyotyping)," provides a comprehensive overview of the karyotyping process, including its significance in diagnosing genetic disorders.
The second video titled "Karyotype" delves into the intricacies of karyotype formation and its relevance in genetic studies.